Process validation includes a number of functions taking place more than the lifecycle with the product and process.
Definition: Future validation is conducted prior to the commercial distribution of a product. It establishes documented proof that a technique or process performs as intended depending on preplanned protocols.
The real-time mother nature of this validation method provides rapid assurance of process reliability. Any deviations or difficulties could be discovered and addressed immediately, lessening the chance of non-compliance.
Developer's guideTechnical documentation for developers.Assist centerAssistance with onboarding and System mastery.
Selected individual from Generation shall ensure the suitability of your equipments shown in the protocol;
Release the PV batches for distribution after: Effective completion of PV activity and overview, approval and signing off the PV interim report with supporting Uncooked information.
In this phase, the process is designed and documented in detail. The significant process parameters and the corresponding running ranges are determined.
Stage 2 – Process Qualification: Through this stage, the process design and style is verified as remaining capable of reproducible industrial production.
Firms that also don't use paperless validation software encounter important problems: the higher fees more info connected to hazard management, validation, and the next modify management and continued qualification to take care of the validation status all over the lifecycle of entities.
The U.S. Food items and Drug Administration (FDA) has proposed guidelines with the next definition for process validation: – “PROCESS VALIDATION” is setting up documented evidence which offers a higher degree of assurance that a certain process regularly creates a product meeting its predetermined technical specs and high quality attributes.
So in the event you’re ready to get a QMS that was purpose-built for medical device businesses like yours, then get your free demo of Greenlight Expert →
In addition, process layout consists of the choice of acceptable machines and facilities that can be Utilized in the output process. Elements like potential, dependability, and compatibility Together with the process specifications are taken under consideration to be certain easy and economical operations.
To educate all personnel linked to get more info the execution of this qualification protocol for pursuing subjects.
Advanced Execution: Specific coordination and adherence to protocols are important to realize responsible results.
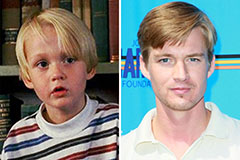 Mason Gamble Then & Now!
Mason Gamble Then & Now! Marla Sokoloff Then & Now!
Marla Sokoloff Then & Now! Susan Dey Then & Now!
Susan Dey Then & Now! Daryl Hannah Then & Now!
Daryl Hannah Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now!